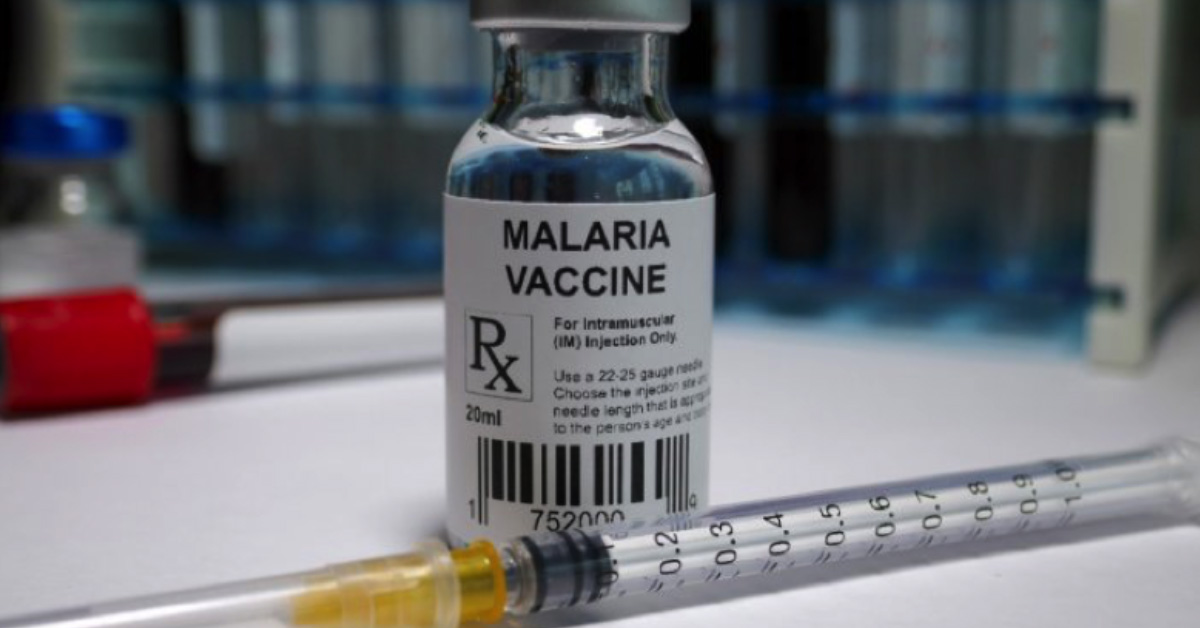
Malaria has been plaguing us since the dawn of human civilisation. We are now finally on the verge of eliminating it – with a vaccine. Watch out, you pesky mozzies!
Showing great promise in early clinical trials, researchers at the Jenner Institute at Oxford University and the Burkina Faso Clinical Research Unit described a new malaria vaccine called R21/MM, with a 77% efficacy. That’s a major leap in efficacy compared to the only malaria vaccine available now, RTS,S/AS01, also known as Mosquirix, which is about 56% effective over a year.
About 400,000 people lose their lives to malaria every year, the majority being young children. According to WHO, 94% of malaria cases happen in sub-Saharan Africa. Malaria-carrying mosquitoes thrive in warm regions across the globe. With climate change, the geographic range of the mosquito will continue to expand steadily. This breakthrough in malaria vaccine technology is fantastic news as it’s the first time we have a potential vaccine that can really help us curb the scourge of malaria.
But what’s the hold up in creating an efficient vaccine for malaria, when in contrast, scientists developed the first COVID-19 vaccines within days?
A tough parasite to crack.
SARS-CoV-2, the virus responsible for COVID-19, has 12 genes. It’s also relatively easy to target the virus’s spike protein for antiviral purposes.
On the contrary, Plasmodium – the culprit that causes malaria – is a parasite. It’s not a bacteria. It’s not a virus. It’s a large organism that floats around in our bloodstream, infecting red blood cells. Researchers have to find some way to alert the immune system and target it. But because it’s such a big organism – with more than 5,000 genes – there are a multiplicity of targets that could be chosen. That’s the fundamental challenge over the years – finding a good target and making sure you get a good enough immune response.
How does the vaccine work?
The new vaccine builds on a previous malaria vaccine to “put more malaria in and less of the carrier protein”, says Adrian Hill, director of the Jenner Institute at Oxford University.
A pre-erythrocytic vaccine, R21/MM is a combination of a more enhanced version of the RTS,S vaccine (R21 in the vaccine’s name) and a vaccine booster, or adjuvant called Matrix-M (MM in the vaccine’s name)
R21 targets a specific protein present on the surface of the Plasmodium parasite in its sporozoite form. The focus on a particular protein produces better-targeted and more consistent immunity than exposing the body to the whole disease agent. Adding to the efficiency of R21 is the fact that the protein that it targets rarely mutates or varies among strains of malaria.
The saponin-based adjuvant, MM, a proprietary invention of Novavax, is an additive that works together with the vaccine to boost the immune system to a higher level. Protein-based vaccines generally require an adjuvant, as the human body will not necessarily react to foreign proteins by inducing a full immune response.
Full steam ahead for vaccine trials.
After the successful phase 2 trial that proved the safety and high efficacy of the vaccine, clinical trials will be moving on to the third phase in four countries across Africa – Mali, Tanzania, Kenya and Burkina Faso. A larger sample size, some 5,000 children, will be involved in tests designed to delve deeper into the safety aspects of the vaccine. This will determine if the good efficacy of the R21/MM vaccine can be replicated across different regions.
Though still a long way from getting phase 3 trial results and then successfully manufacturing the vaccine at scale, this latest development could potentially drive a nail in the coffin of malaria in many countries. Being the first to surpass the WHO’s threshold of 75% efficacy for a malaria vaccine, the big increase in vaccine effectiveness also means that we can prevent three quarters of malaria cases among vaccinated children. This equates to hundreds of thousands of lives each year.
Opportunity to empower Africa with vaccine self-sufficiency.
If things go well and after regulatory approvals, the vaccines will need to be made somewhere, most likely at the Serum Institute of India, where collaboration is currently underway with Oxford and Novavax for the phase 3 trials.
Stimulating vaccine manufacturing in Africa is also being discussed. It’s home to about 1.2 billion people, but imports 99% of the vaccines it uses. One of the main challenges of creating new facilities to make vaccines is the need for an ongoing business model, a plan on what can be made year in year out. The malaria vaccine seems to be a prime candidate for something that could be manufactured on the continent.
Perhaps foundations such as the Gates Foundations, Wellcome Trust or The Global Fund will get together with business institutions to consider the case for accelerating health security in Africa, not only for malaria, but also in preparation for future pandemics.