You’ve probably heard of ultrasound. And the first image that comes to mind is most likely someone pregnant sitting in a medical exam room, gleefully looking at the developing baby on a monitor.
While this is a fairly common and useful application of ultrasound technology, which utilises high-frequency sound waves, it’s actually not its only function.
These magical sound waves can also be used to manipulate the chemical properties of materials.
Yep, you heard that right!
Our Science Writer takes you on a journey through his area of expertise — power ultrasound.
Hear me out!
Humans can hear frequencies in the range of 20 to 20,000 hertz (or 20 kilohertz).
Above that frequency, we’d enter the realm of ultrasound — 20 kilohertz to 200 megahertz.
Power ultrasound is located at the lower end of the range, and this is where the magic happens. Ultrasound waves generate very unique properties in liquids when their frequency lies in the range of 20 – 100 kilohertz. Did you know that dolphins can hear sounds up to 100 kilohertz?
As a comparison, medical or diagnostic ultrasound — the one used to produce images of fetuses — utilises sound waves located at the higher end of the range (about 2 – 18 megahertz).
Miniature suns in liquids.
When applied to liquids, power ultrasound can alter the chemistry of the liquid itself or the materials present in the liquid.
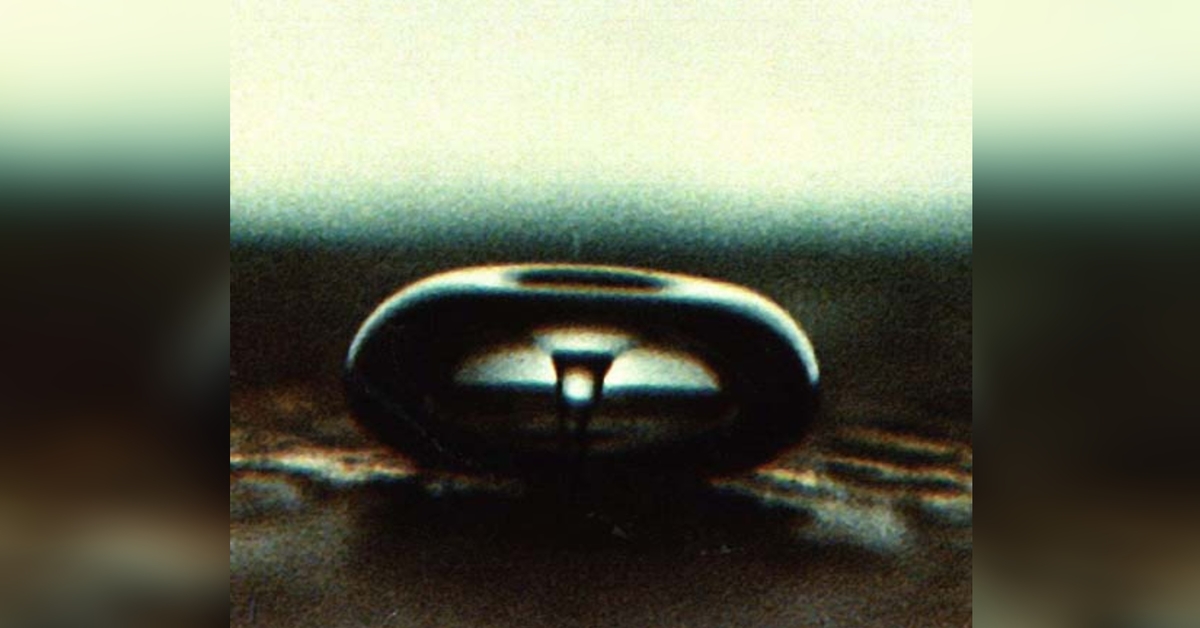
The ultrasound waves don’t directly interact with the molecules to induce the chemical change. Rather, the energy from the waves causes a phenomenon known as acoustic cavitation, in which many tiny micro-bubbles form in the liquid. These bubbles manifest as a result of the voids created by the compressive and expansive forces from the ultrasound waves when they interact with the liquid.
And here’s where things get utterly strange.
Under the right conditions, the cavitation bubbles will then collapse and release enormous amounts of energy. We’re talking about temperatures rivalling that of the Sun and pressures as immense as those experienced at the bottom of the Mariana Trench!
The collapsing bubbles, or “hot-spots”, are localised and adiabatic, meaning the massive build-up of energy is confined within the bubbles throughout microscopic regions of the liquid. So you don’t really end up with an actual sun in your beaker!
Nevertheless, these “hot-spots” are capable of creating extreme and eccentric physical and chemical environments in the liquid, leading to the acceleration and enhancement of myriad chemical reactions.
Such a phenomenon is observed in nature too. A mantis shrimp’s punch is so explosive that it doesn’t just kill its prey in one single blow, it generates lots of cavitation bubbles during the strike as well. The “hot-spots” are so intense that they even emit faint light! (Through a process called “sonoluminescence”)
With 1500 Newtons of punching force (what’ll you need to bench press 150 kilograms), you probably don’t want to mess with this little guy. Let’s just say that in the labs of grad students, these miniature heavyweight strikers have broken aquariums, smashed glassware and split thumbs… Ouch!
Ultra-useful tool to manipulate chemical reactions.
From speeding up metal recovery from wastes to altering catalyst properties, power ultrasound has been incorporated into various chemical processes for many years.
While many laboratory investigations show very positive results, the major challenge for this technology lies in its feasibility to be implemented on a broader industrial scale — it’s relatively expensive to operate large ultrasonic devices and they’re intense guzzlers of energy.
But researchers are actively optimising the design of large ultrasonic processes to drive the operating costs down, transforming the technology into a sound solution for chemical applications.