Iron is one of the most important metals in the world. It’s strong, lightweight and malleable, making it a great material for many objects from gigantic bridges to nuts and bolts.
The Achilles heel of the metal is its susceptibility to corrosion. In the presence of air and moisture, iron is oxidised to form rust, severely jeopardising the structural integrity of the metal. To prevent this, the industry galvanises iron with zinc, or plates its surface with nickel, or dopes iron with a concoction of metals, forming stainless steel.
The renewable energy industry, however, is beginning to love the brown stuff. Why? Rust literally gives renewables more power, in the form of iron-air batteries.
The iron-air battery might be a technological advancement that could potentially diversify the energy storage industry. But with most engineering breakthroughs in the industry ending up being less than what meets the eye, let’s take a closer look at how the battery works and see if it could turn up to be a realistic disruptor in the grid-scale energy sector.
Rust is a must in iron-air batteries.
Iron-air battery technology has actually been around for decades, but it has only recently gained momentum, driven by incentives to develop low-cost, environmentally benign and robust rechargeable batteries.
The battery’s working principle is breathtakingly simple, which can essentially be encapsulated by two words – reversible rusting.
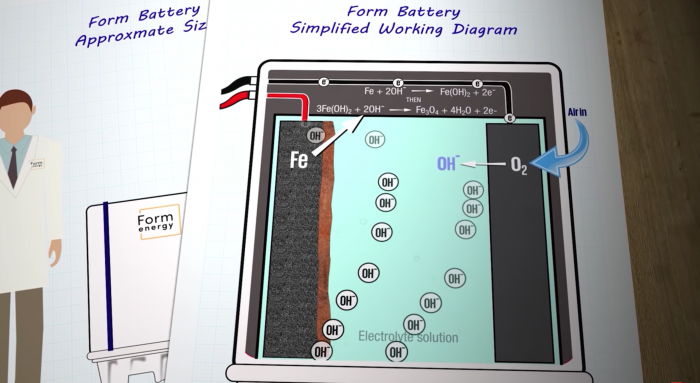
Simple working diagram of an iron-air battery. Photo credits: Just Have a Think/YouTube
Each cell in the battery has an anode, consisting of pellets of metallic iron on one side, and an air-breathing cathode on the other side. Both electrodes are immersed in a water-based non-flammable electrolyte, much like what you’ll find in a standard AA battery.
As the battery discharges, oxygen from the air reacts with the iron via the liquid electrolyte. The reaction reduces the air to hydroxide (OH-), which then oxidises the iron to iron oxide, forming rust on the surface of the anode. During the process, electrons are released and electricity is generated.
To recharge the battery, an electrical current – ideally generated from renewable sources such as wind or solar – is run through the cell, reversing the reaction. This liberates the oxygen from the rust, turning it back into iron. And Voila! A recharged iron-air battery!
Another one bites the rust.
Do iron-air batteries have what it takes to edge out other more commercial batteries such as lithium-ion (Li-ion)?
When it comes to energy density, the iron-air battery (764 watt-hours per kilogram) is at a much greater advantage than the Li-ion battery (100 – 265 watt-hours per kilogram). The reason lies in the fundamental chemistry of the batteries: Iron-air batteries are run by electrochemical reactions, while Li-ion batteries are governed by the process of intercalation.
In a Li-ion battery, lithium ions move back and forth in an electrolyte and fit into empty spaces inside the crystal structures of the electrodes. This means that the capacity of the battery is limited by the physical volume of the electrodes. Conversely, an iron-air battery doesn’t move metal ions between the electrodes. The electrochemical reaction simply deposits rust on the surface of the iron electrode, which means that the only limiting factor is the surface area of the electrode. As a result, the mass requirements of the battery is greatly reduced, leading to a much higher theoretical energy density.
Costs of Li-ion batteries are also largely dependent on the prices of the myriad materials they use such as nickel, manganese, cobalt and lithium. The latter element, roughly 2,800 times less abundant than iron, is already sparking a ‘market war’ as the grid becomes increasingly electrified.
Being one of the five most plentiful elements on Earth, iron could provide a much cheaper alternative to Li-ion batteries. Boston-based Form Energy claims their iron-air batteries could provide energy storage at one-tenth the cost of Li-ion batteries – about $20 or less per kilowatt-hour. This price reduction is substantial as studies show that the energy capacity cost of long-duration storage technology must fall below $20 per kilowatt-hour to reduce total carbon-free electricity system costs by at least 10 %. Form Energy’s 300-megawatt prototype is planned to go live in 2023 and they intend to prove that the technology is viable on a large scale.
While iron-air batteries are too heavy for mobile phones or battery electric vehicles, we’ll have to wait and see if the grid energy storage technology is economically feasible. Time will tell if iron-air batteries are truly capable of solving one of the most elusive problems plaguing renewables: Cheaply storing large amounts of electricity to power grids when the sun isn’t shining and the wind isn’t blowing.