While some scientists are looking outward to study the possibilities of life on other planets, some are peering inward to investigate the fundamental mechanisms of life, as we know it.
There’s still much we don’t know about the human body. But since the advent of advanced supercomputing, the learning curve for understanding how our body works has been sped up significantly.
This time, researchers have harnessed the computing power of supercomputers to decipher how energy is transmitted across the powerhouse of the cell – the mitochondrion.
Say hello to the power plants of your cells.
The mitochondrion is known as the powerhouse of the cell as it’s responsible for producing energy from the food we eat, through a process called cellular respiration. Energy from this process is released in the form of adenosine triphosphate (ATP) – the energy currency of the cell.
As the saying goes: It takes two to tango. This is particularly true when it comes to how cells operate. Protein molecules inside a cell ‘dance’ with other protein molecules in an intricate choreography, responding to cellular signals and regulating each other’s cellular activities.
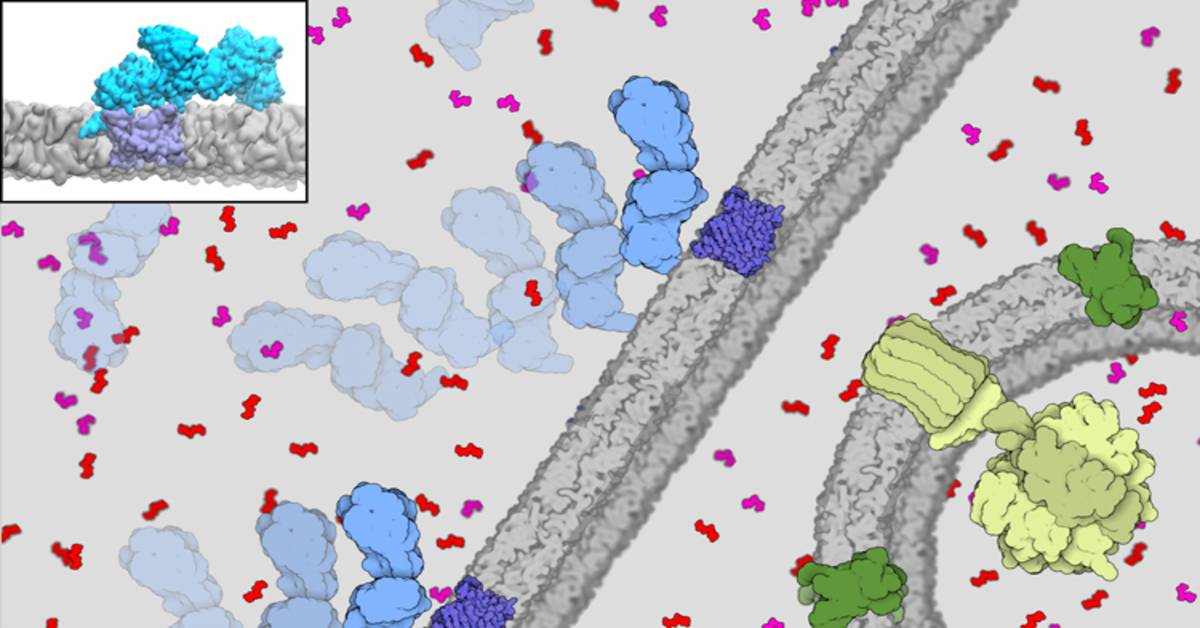
To transport ATP to the power-hungry parts of the cell, an intimate dance is initiated between protein enzymes called hexokinase-II (HKII) and proteins in the voltage-dependent anion channel (VDAC) found on the outer surface of the mitochondria. The HKII enzymes bind with proteins on the VDAC, gobbling up ATP and converting it into fuel used to energise cells.
Scientists know that these proteins bind with each other, but the million-dollar question is HOW? In a recently published study, scientists have finally found the answer.
Supercomputers make the impossible possible.
Thanks to the Stampede2 supercomputer at the Texas Advanced Computing Centre (TACC), researchers were able to simulate how the HKII proteins bind with the VDAC proteins. With 18 petaflops of peak performance, the supercomputer helped the researchers to develop the most detailed and sophisticated model yet of the complex formed by the binding of HKII and VDAC, resulting in a system size of about 700,000 atoms.
The modelling was broken down into three parts. Using all-atom molecular dynamics and simulation models, the first part studied how HKII binds to the outer membrane of the mitochondria. Next, the researchers used Brownian dynamics to investigate how HKII drifts on the membrane to meet the VDAC proteins. Lastly, they zoomed in on the interactions to get a more refined and specific model of this particular protein-protein interaction, resulting in the most stable complex of the two proteins formed.
Studying these complex biological interactions usually require long simulations to be scientifically convincing. Without advanced computing resources, scientists wouldn’t have been able to conduct experiments on such a scale to improve our knowledge of cellular systems.
Zooming into cells to see the bigger picture.
This fundamental research is akin to a computational microscope that allows one to deeply study cellular systems and processes at the molecular level. Through this type of research, scientists have a better picture of how cellular molecules come together, how they move and how their structures evolve to carry out a particular function that can only be measured indirectly via experiments.
High-performance computing is one of the precursors to the success of such research. Coupled with the development of advanced bioinformatics tools, researchers will be able to unveil the molecular basis of major diseases, such as cancer, and enhance the speed of drug discovery.