Perfluoroalkyl and polyfluoroalky substances, or collectively known as PFAS, are nasty stuff.
Invented in the 1930s, PFAS have the distinguishing structure of a chain of carbon atoms with at least one fluorine atom attached. And the exceptional strength of that carbon-fluorine bond is exactly where the problem lies.
Like a double-edged sword, the strong bonds render PFAS highly resistant to heat, oil, stains, grease and water – a wonderful coating material for clothing, furniture, adhesives and food packaging – but that also means that PFAS do not break down in the environment and can even build up in animals.
And that’s bad news for us. Studies have uncovered that long-term human exposure to PFAS contributes to kidney and testicular cancer, impacts the immune function and messes up the metabolic and endocrine system.
But removing them from the environment is one hard nut to crack. Scientists have long tried to use chemical treatments to get rid of them, but mostly to no avail.
Now, they’re finally starting to see the light at the end of the tunnel, and in the light is a silhouette of a silicon chip…..
Supercomputers help see the light.
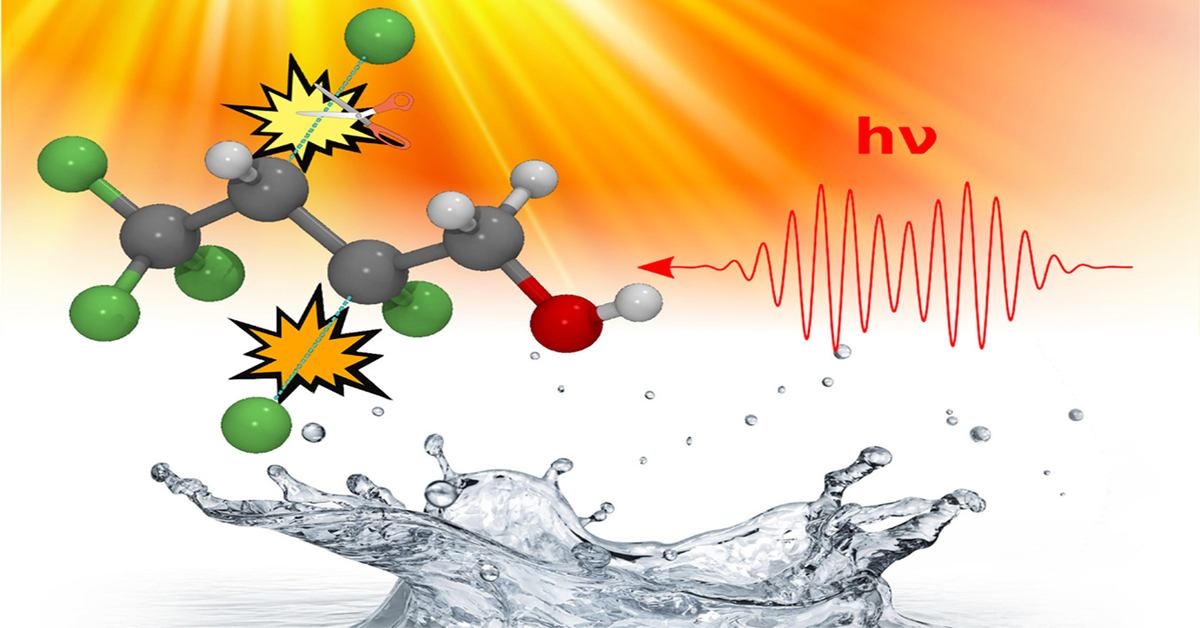
In a published study, researchers at the University of California Riverside (UCR) were surprised to learn that electromagnetic radiation, or light, could be used to efficiently degrade the carbon-fluorine bonds in PFAS contaminants.
The fundamental mechanisms underpinning the light-induced decomposition process were previously poorly understood. But the scientists were able to rapidly converge to a solution with the help of supercomputer-aided simulations.
Harnessing the data-crunching ability of the Comet supercomputer at the San Diego Supercomputer Centre, they were able to shine a light on the degradation process at a quantum-mechanical level of detail, enabling them to further devise creative approaches for directly eliminating PFAS.
Turbocharging environmental research by orders of magnitude.
To bombard virtual PFAS dissolved in virtual water with virtual water, the scientists ran a series of simulations based on the real-time, time-dependent density functional theory (RT-TDDFT), available on GitHub.
Hardware-wise, the Comet supercomputer that made the research possible provided a peak performance of 2.76 petaflops, powered by 1,944 Intel Xeon E5-2600 v3 CPU nodes and 72 Nvidia K80 and P100 GPU nodes.
After more than 570,000 CPU hours and efficient code-compiling, the research team successfully performed a series of iterative calculations to work out exactly what frequency of light is capable of breaking the incredibly strong carbon-fluorine bonds in PFAS. And breaking these bonds means destroying the molecules, making them safer and less toxic.
Moving forward, the research team is looking to accelerate PFAS remediation efforts through their RT-TDDFT approach, which is proven to be a viable, direct and simple technique to destroy PFAS contaminants in critical applications such as water purification. On another note, they also see the opportunity to marry high-performance computing (HPC) – provided by supercomputers – with RT-TDDFT to swiftly discover more catalytic materials for the degradation of PFAS and other environmental contaminants.
Clouds with a supercomputing lining.
Today’s cloud comes with a supercomputing lining, which enabled the potentially life-saving research work conducted by the UCR scientists. They’re empowered with the capability to run their simulation software of choice on massive cloud-based computing power, without having to sweat the infrastructure.
Just as cloud computing revolutionised the enterprise and created new ways for businesses to engage with their customers, supercomputing will open up new possibilities for innovation breakthroughs by turbocharging R&D by orders of magnitude.
Supercomputing in the cloud is beginning to underpin innovation in many industries, and it is making possible what seemed like science fiction yesterday.