Heavy industries such as cement, steel and chemicals and heavy-duty transport such as road trucking, shipping and aviation produce nearly one-third of the world’s greenhouse gas emissions, and reducing that number is a monumental challenge.
Hydrogen is playing an increasingly crucial role when it comes to making those hard-to-abate sectors a thing of the past, serving as a crucial piece of the net-zero by 2050 puzzle. It has the potential to store surplus renewable energy, decarbonise heavy transportation and serve as a zero-emission energy carrier.
However, storing hydrogen effectively is still an art that is being perfected as conventional high-pressure or cryogenic storage can pose significant technical and engineering challenges.
Metal hydrides offer an alternative.
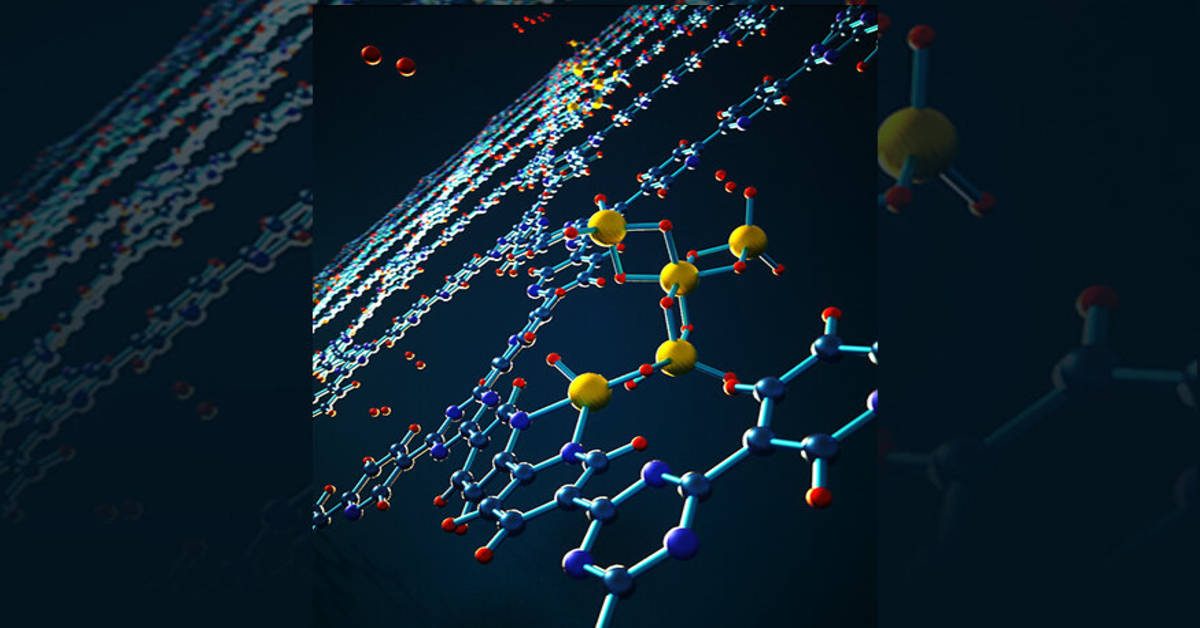
Overcoming these material challenges requires researchers to think outside the standard hydrogen box. Sandia National Laboratories (SNL) and Lawrence Livermore National Laboratory (LLNL) scientists turned to metal hydrides, metallic compounds that chemically bond with hydrogen molecules, stabilising the molecules for storage. They’re known for their exceptional energy densities, as well as their ability to adsorb and release hydrogen under mild conditions.
Solid metal hydrides have the ability to pack more hydrogen in terms of volume and mass compared to traditional gas-phase, pressurised hydrogen storage tanks.
But they too suffer from several drawbacks such as the poor thermodynamics of hydrogen adsorption, meaning it’ll be extremely difficult to regenerate the metal hydrides after first use, more often requiring extremely high hydrogen pressures to do so. Consequently, the real-world applications of metal hydrides are yet limited.
Enter the nanoworld.
In their research, the SNL and LLNL scientists focused on a common metastable metal hydride called aluminium hydride, or alane, which can store twice as many hydrogen molecules as those present in liquefied hydrogen.
The ingenuity of their research lies in the usage of a highly porous covalent triazine framework — a new and innovative nanoporous organic material that provides high chemical bonding and stability — to grip onto the alane molecules so tightly that they can’t escape.
 Entering the nanoworld is where things get freaky. When bulk materials are reduced to their nanoscale, the resulting nanomaterials present chemical and physical properties that differ greatly from their bulk. Take sunscreen as an example. It contains titanium dioxide nanoparticles that block ultraviolet radiation just as effectively as normal-sized titanium dioxide particles. But as the nanoparticles hardly scatter any visible light due to their extremely small size, you’re saved from having your face painted white.
Entering the nanoworld is where things get freaky. When bulk materials are reduced to their nanoscale, the resulting nanomaterials present chemical and physical properties that differ greatly from their bulk. Take sunscreen as an example. It contains titanium dioxide nanoparticles that block ultraviolet radiation just as effectively as normal-sized titanium dioxide particles. But as the nanoparticles hardly scatter any visible light due to their extremely small size, you’re saved from having your face painted white.
Harnessing the wonders of nanomaterials, the researchers stabilised alane within the nanopores of the framework, flipping the thermodynamics of alane upside down. Ultimately, the regeneration capabilities of alane are tremendously improved compared to its bulk counterpart, allowing a ten-fold decrease in regeneration pressure from 6,900 to 700 bar (or 100,076 to 10,153 psi).
Innovation to the rescue.
This innovative work paves the way for developing composite materials applicable for real-world hydrogen storage. For instance, a pressure of 700 bar (10,153 psi) is readily achievable in commercial hydrogen fueling stations, so vehicular hydrogen storage tanks of future hydrogen cars could be made out of alane.
Furthermore, metal hydride storage tanks can also operate at much lower pressures compared to conventional storage tanks. To store one kilogram of hydrogen, a typical high-pressure gas tank operates at 1000 bar (14,504 psi), while its metal hydride counterpart could operate at 40 bar (580 psi). This low-pressure solution puts you on the safe side while effectively storing energy.
Though more improvements have to be made, this study could also have further implications for tuning the properties of other energy-generation and storage materials, including batteries and catalytic materials.