When humans invented the first optical microscope in the 1600s, the best achievable magnification was only around 270x.
Optical, or light microscopes use light and a series of magnifying lenses to uncover the hidden world of cells. But that means anything smaller than the wavelength of light would be invisible to the naked eye.
Enter the world of electron microscopy, where electrons with wavelengths 100,000 times shorter than that of visible light are used instead of a light source. Invented in the 1930s, typical electron microscopes are capable of peering into a panoply of biological and inorganic specimens such as microorganisms, cells, metals and crystal structures, with magnifications of up to 10,000,000x.
But electron microscopes, too, hit a bottleneck. Increasing the resolution of images requires raising the electron beam’s energy. This spells disaster for samples, particularly those of biological nature, as the energy would become so enormous that it’s destructive.
Electrons get funky.
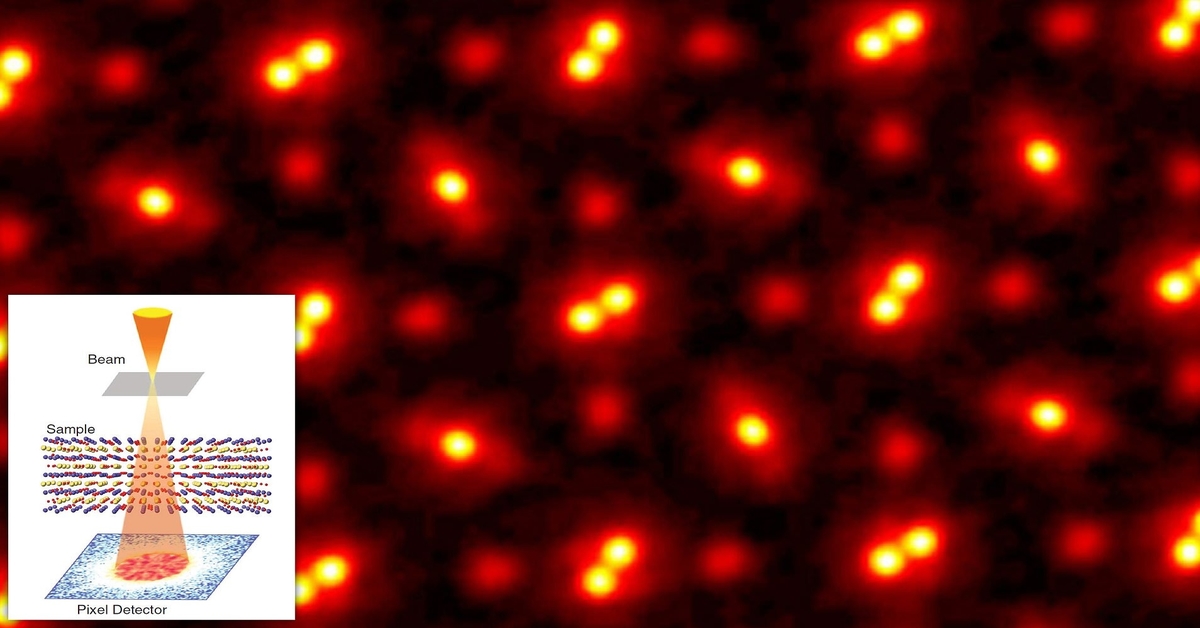
Enter the world of ptychography. This technique uses electrons too, but with a chaotic twist. Eliminating the electromagnetic lenses found in standard electron microscopes, a ptychographic microscope fires billions of electrons per second at a target material from multiple angles. Researchers then watch how the material scatters the electrons — sometimes the electrons pass through cleanly; other times they collide with atoms and bounce around inside the material before leaving. Based on the array of patterns generated by the electrons as they hit a detector, advanced algorithms can work out the location of atoms in the material and their shapes, therefore producing an image of the material while preserving its pristine state.
Using this microscopy method, Professor David Muller and his research team captured the highest-resolution image of atoms to date, with an impressive magnification of 100,000,000x. They even bested their own Guinness World Record in 2018, where they also achieved the highest microscope resolution at that time.
It’s a showcase of the power of advanced algorithms and high-performance computing in breaking the physical limitations of microscopes.
The bigger picture.
Now, instead of just one to a few atoms thick, Professor Muller’s group can use electron ptychography to capture multiple layers tens to hundreds of atoms thick. This makes the technique much more relevant to materials scientists, particularly to those who study nanomaterials and beyond.
As silicon-based computer chips are inching closer towards the atomic scale, being able to see what’s going on at the atomic level is essential to developing next-gen electronic devices. This could greatly accelerate the search for alternative materials to replace silicon for power-saving semiconductors that pack a larger computational punch, as well as improve imaging techniques for materials used in quantum computers.
Furthermore, the battery-making industry can also benefit from this high-resolution microscopy technique. Scientists can peer into chemical reactions in much finer detail to study how different materials interact with one another, thus engineering safer and more efficient energy storage systems that are crucial for the transition from fossil fuels to renewable energies.
But is this landmark study the ultimate limit of how far we can look into the fundamental building blocks of objects?
In the picture supplied by the research group (hero image), you can observe a blurry halo surrounding the individual atoms. The blurriness isn’t an error in the detector or interference from the air — it’s the jiggling of the atoms themselves, due to the heat from the electrons.
So unless there’s a method to cool and secure the atoms in place and observe them in an entirely new way, perhaps this is as good as it gets!
* Editors note — I suspect we will do better, science is like that 🙂